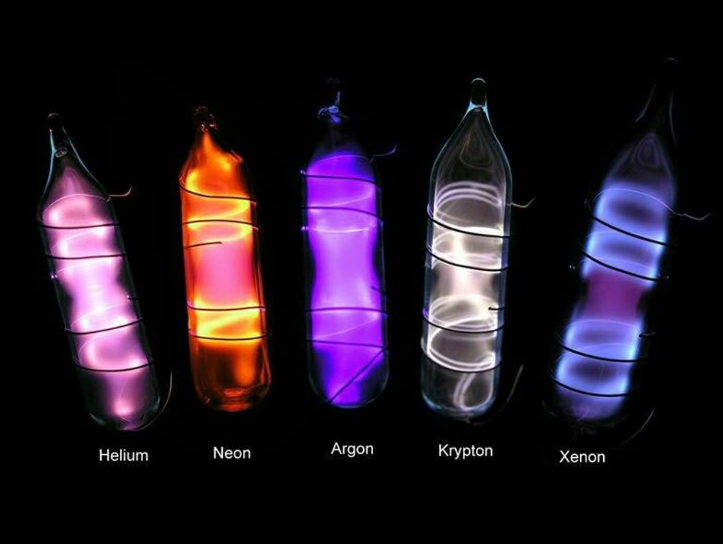
In the world of prepping and survival, every piece of gear is scrutinized for its utility, durability, and multi-purpose potential. We think about water filters, fire starters, and communication devices. But what if I told you that the vibrant, glowing tubes of noble gases you see in the image—Helium, Neon, Argon, Krypton, and Xenon—hold a fascinating, albeit niche, place in the arsenal of a forward-thinking prepper? While you won’t be building one in your bug-out bag, understanding the principles behind them can unlock critical insights into illumination, emergency signaling, and even the subtle art of atmospheric awareness in a grid-down scenario.
Beyond the Glow: Noble Gases in a Survival Context
At first glance, these beautiful glowing tubes might seem like a scientific curiosity, far removed from the grit of survival. However, their unique properties touch upon several vital areas for preppers:
- Reliable Illumination & Signaling: The core concept is light generated by gas ionization. In a world without readily available electricity, sustainable light sources are paramount. While a modern survivalist won’t be carrying a noble gas discharge tube, the principle informs us about how light can be created without combustion (like a candle) or a complex bulb filament. This points to understanding alternative light technologies that might emerge or become more relevant in a long-term grid-down scenario, such as electro-luminescent panels or even understanding the potential of various phosphorescent materials. For emergency signaling, the distinct colors and brightness of these gases (if we could easily power them) would make for unmistakable visual communication.
- Atmospheric Monitoring: While you won’t be using a noble gas tube to detect toxic gases directly, the fact that different gases react distinctly to electrical currents is a powerful concept. In a survival situation, understanding the composition of the air, especially in enclosed spaces or after a catastrophic event, could be life-saving. The distinct “fingerprint” each noble gas gives off when energized indirectly highlights the importance of tools that can differentiate atmospheric components—whether that’s a simple CO detector or more advanced (though less accessible) gas analysis tools.
- Resourcefulness & Ingenuity (The DIY Spirit): While building a noble gas discharge tube from scratch is beyond the scope of most preppers, the image itself embodies the spirit of scientific understanding and resourceful creation. In a post-collapse world, the ability to understand fundamental scientific principles and adapt existing technologies (or create new ones from scavenged parts) will be invaluable. Learning how light works, how gases react, and how to harness energy, even in its most basic forms, moves you from a consumer of technology to a potential creator.
Not a DIY Project, But a Mindset
Let’s be clear: You cannot and should not attempt to “make” these tubes in a survival scenario. Creating sealed glass tubes with specific noble gases requires high-vacuum systems, precision gas handling, and high-voltage electronics—all resources that will be scarce or non-existent when the grid is down. Attempting to do so without proper training and equipment is extremely dangerous.
Instead, let these glowing tubes inspire a different kind of prepping:
- Study the Fundamentals: Understand basic physics and chemistry. How does light work? How do batteries store energy? What makes certain materials glow? This knowledge is infinitely more valuable than any single piece of gear.
- Explore Practical Alternatives: Focus on robust, low-tech, and sustainable light sources. Think hand-cranked lanterns, solar-powered lights, and a deep understanding of firecraft.
- Emergency Signaling: Learn various signaling techniques—mirror signals, flag signals, and different methods of creating visible light at night that can attract attention.
- Air Quality Awareness: Invest in reliable, battery-powered carbon monoxide detectors for any enclosed survival shelter.
The noble gas tubes are a testament to the wonders of science and the power of harnessed energy. While they won’t be a direct tool in your bug-out bag, they serve as a potent symbol for the prepper’s mindset: understanding the world around you, seeking innovative solutions, and being prepared to illuminate the darkness, both literally and figuratively, when the lights go out.
The Science Behind the Glow: How Noble Gases Light Up
Noble gases (Group 18 of the periodic table) are known for being extremely unreactive. However, when you introduce a strong electrical current, something fascinating happens. The electricity “excites” the electrons in the gas atoms, causing them to jump to a higher energy level. When these electrons fall back to their original, more stable energy level, they release the excess energy as a photon of light. The specific wavelength (and therefore color) of the light is unique to each element, determined by the electron shell structure of its atoms.
- Helium glows with a distinctive pale pinkish-white or purplish color.
- Neon produces the familiar fiery red-orange glow, the same color seen in classic “neon” signs.
- Argon emits a soft lavender or bluish-purple light.
- Krypton has a bright whitish or grayish-white glow.
- Xenon shines with a beautiful, rich blue-violet light.
How to Create Your Own Noble Gas Discharge Tubes
Disclaimer: Creating sealed gas tubes requires specialized equipment and knowledge of high-vacuum systems and high-voltage electronics. This is not a project for a beginner and should only be attempted by a professional or someone with a strong background in chemistry and electrical engineering. The following is a conceptual overview of the process, not a step-by-step guide for home use.
Step 1: The Glass Vessel 🧪
The process begins with a clean, empty glass tube, often with a narrow neck to connect to the vacuum system. This is the container for the gas. It must be free of any impurities that could affect the color or the vacuum.
Step 2: The Vacuum System 🌬️
A powerful vacuum pump is used to evacuate all the air from the tube. The goal is to create a high-quality vacuum (as close to absolute zero pressure as possible) to ensure no other gases interfere with the noble gas.
Step 3: Purging and Filling the Tube ⚛️
The tube is filled with a small amount of the desired noble gas and then evacuated again to remove any lingering air. This “purging” process is repeated several times. Once the tube is completely evacuated, a small, precise amount of the pure noble gas is introduced, bringing the pressure to a low, specified level.
Step 4: Sealing the Tube and Adding Electrodes 💡
Electrodes (typically metal wires) are sealed into the ends of the tube to allow for the electrical current to pass through. The glass is heated and sealed around the electrodes, creating a permanent, airtight seal.
Step 5: Applying the High Voltage ⚡
Once sealed, the tube can be connected to a high-voltage power supply. When the power is turned on, the voltage difference between the two electrodes ionizes the gas, creating a plasma that emits light.
The tubes shown in the image are beautiful examples of this scientific process in action. They serve as a powerful reminder that even the most unreactive elements can reveal stunning properties under the right conditions.